Water is easily overlooked until it is absent. In other words, it is often the forgotten ingredient and not the prioritized parameter to consider when production problems arise. Reduction in water intake can be the first indication of health problems. Therefore, water availability and quality are extremely important for animal health and productivity.
Do you know that water is the most important nutrient for animals?
Water is involved in all basic physiological functions
- temperature control
Water is able to store a large amount of heat in liquid form and then lose heat upon evaporation. This makes it extremely important in temperature regulation. Water aids in the removal of excess heat from the body
- digestive processes
is involved in digestion and metabolism of nutrients and in the elimination of waste materials.
- excretion
Animals' bodies consist mostly of water
- Approximately 70-80% of a piglet’s body is water.
- Approximately 55%-75% of poultry's body is water
- Water represents around 65% of the weight of the egg
Water is consumed in larger quantities
- Many factors can influence water intake, such as, environment, accessibility, animal physiology and health status.
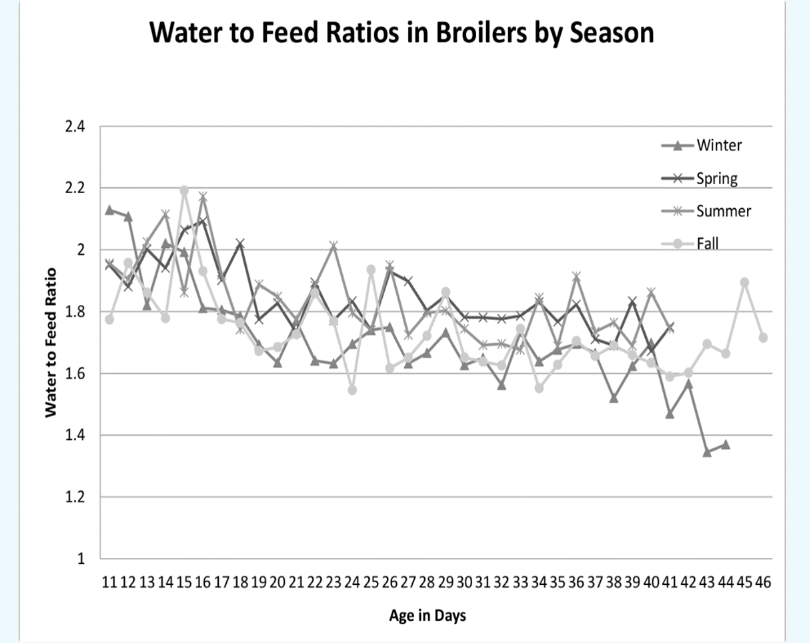
Water to feed ratios in broiler by season (Source: McCreery, D. Water consumption behaviour in broilers. Agricultural and Food Sciences. 2015.)
- As a rule of thumb, broilers and pigs drink 2-3 L of water for 1 kg of feed intake.
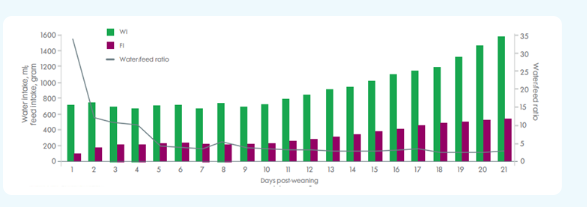
Relation between daily water and feed intake post-weaning (days 0-21) (Source: Fleuren, M. et al. Water intake of newly weaned pigs. Is there a link with (gut) health? Zero Zinc Summit 2022.
Effect of water quality on animal performance and health
First and foremost, it is important that animals have access to clean, fresh, and safe water at all times. This essential resource is typically provided through nipples, cup drinkers, or troughs. It's crucial to note that the flow of water and the height of the nipples or cups play a significant role in the amount of water consumed by animals.
The most common water quality challenges affecting animal production include high concentrations of minerals, sulphates, nitrates and nitrites, bacterial contamination, growth of blue-green algae, and chemical contamination associated with agricultural and industrial activities.
Water quality also depends on its source and poor hygiene when storing it can represent a health concern.

Have you heard about biofim?
Do you know that slime in the internal surface of the pipes? That is biofilm.
Biofilm is an accumulation of microorganisms and (in)organic matter bound by extracellular polymer substances attached to the inner surfaces of pipes and storage tanks in water systems. Biofilm itself provides an environment for bacterial replication.
Controlling biofilm is an important step in keeping good quality water. Flushing water through pipes combined with a good disinfection protocol can be effective in removing/controlling it.
Can low quality water affect the growth and health of poultry?
Limiting water availability to animals will depress production, and poor-quality water is often a factor limiting the intake. Considering that water is consumed in large quantities, if water quality is low, there is an increased risk that water contaminants could reach a level that may be harmful. If the water quality is compromised, not only performance if negatively affected but also health and welfare.
Water intake in the first-second weeks of broiler production is low in relation to the volume of plumbing systems, creating slow flow rates, while environmental temperatures are often high to prevent chilling of the animals. This creates ideal condition for bacterial multiplication in slow‐moving, warm water. High bacterial challenge from contaminated water at this time of life can lower growth rates, decrease water/feed intake and result in scour/wet litter depending on contamination type.
Nonetheless, broilers will survive and even grow with inadequate water intake, but not to their potential.
Monitoring water quality

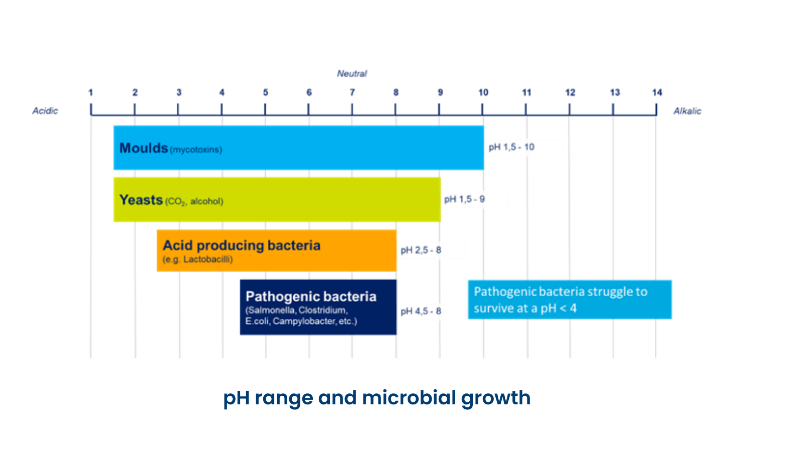
pH
pH
- <7 = acidic
- >7 = alkaline
- < 4 = pathogenic bacteria struggle to grow
pH can affect chlorination, antibiotic/vitamins stability, vaccination efficacy

Hardness
Hardness
- Total divalent ions present in water. Ca and Mg are the most common
- The unit is ppm per CaCO3 equivalents
- “Hard water” has higher CaCO3 equivalents than “soft water”
Hard water is not harmful to animals. However, in the long term, it can clog the drinking nipples. Sodium carbonate can be used to soften hard waste.

Alkalinity
Alkalinity
Ability of water to keep its pH stable. It mainly occurs due to the presence of carbonates in water

Microbiology
Microbiology
Yeast, mould, Enterobacteria and coli are the initial screening test for microbial analysis.
If higher levels, the source of contamination needs to be treated.

There is no direct relation between hardness and pH - hard water does not necessarily have a high pH, and soft water a low pH.
Alkalinity and hardness differences and similarities:
- Alkalinity is the capability of water to resist pH changes or total amount of bases present in water. Hardness measures the total amount of divalent saltsch
- The unit of measurement is the same (ppm in CaCO3 equivalents)
- Both come from limestone or dolomite sources in nature. When the water passes through rocks, it takes minerals. Limestone and dolomite dissolve in water, Ca and Mg ions, and carbonates are mixed with water. Ca and Mg ions cause water hardness, and the alkalinity occurs due to carbonates
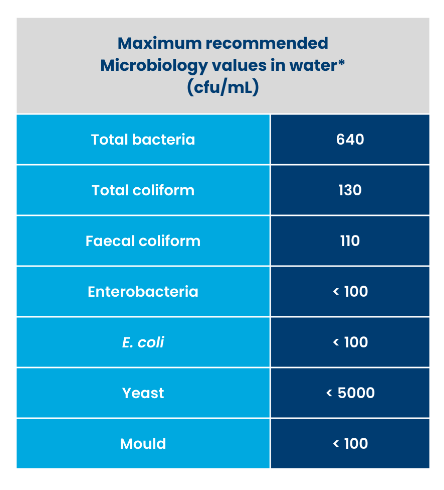
It is important to follow the physiochemical/microbiology water guidelines recommended by the farm vet and/or local authorities.
*Based on different sources.
Water quality may change over time, and therefore one should not rely on past analysis. Water testing should be done routinely (at least once a year, twice is advisable), whereas any unusual changes in organoleptic properties, animals eating or drinking habits, performance or health should immediately trigger the need for water testing.
For water sample collection, always, collect at entrance of the water line, and the last nipple drinker of the system, as contamination through the line changes (see table on right). Allow water to run for a few minutes to obtain a representative sample. Keep samples refrigerated and send them to a lab as soon as possible. Also collect a sample at the water source (i.e., tank), as this gives insight into the contamination within the water system.
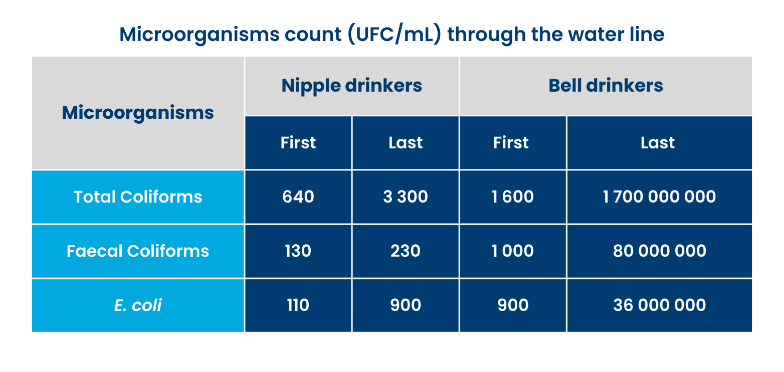
Table: Microorganisms count (UFC/mL) through the water line. Adapted from: Macari, M. and Amaral, L.A. Importancia da qualidade da agua na criacao de frangos de corte: tipos, vantagens e desvantagens. Anais da Apinco. 1997.
Manage water quality
When lab analyses show that microorganisms count is above the maximum recommended, a cleaning of the water system is needed. Cleaning is also advised after use of medication or other additives with risk for biofilm development.
General recommendation:
- Flush the drinking water system with clean water (pressure 2 to 3 bar). Flushing water through pipes can be effective, especially in addressing well-developed biofilms from smooth pipe interiors, such as PVC.
- Use hydrogen peroxide for 24-48 h to loosen the biofilm (empty house). Hydrogen peroxide is more effective in removing biofilm than chloric products. Chloric products are very effective in preventing biofilm formation.
Do not forget to clean storage tanks.
Treatment of the water line in houses with animals is possible but requires suitable chemical agents. Lower concentrations of some chemicals are well tolerated and can slowly remove biofilm without detriment to the animals or to water flow.
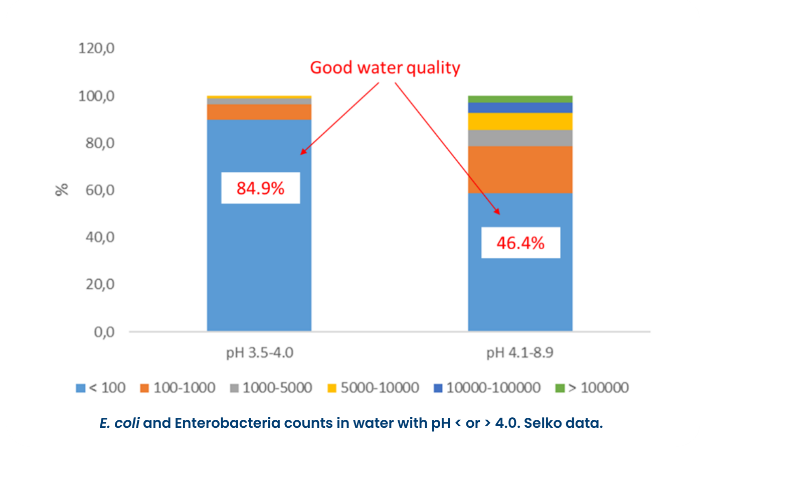
There are many water treatments available: filtration, chlorination, acidification, among others. Chlorination and acidification are the most used methods to keep a good water quality.
Chlorination relies on the effects of hypochlorous acid to kill bacteria and other microorganisms. Free chlorine at 3-5 ppm residual, kills E. coli in 10 seconds. If the bacteria are exposed for less time or at lower levels, they may still be present when the water is consumed. In a farm, bacteria are introduced into the water on an ongoing basis. Maintaining a chlorine residual of will keep these bacteria in check.
For clean water, 3-5 ppm free chlorine provides adequate microbial control in a short contact time. ORP readings will be around 650–700 mV. At this ORP value, pathogenic bacteria such as E. coli or Salmonella are killed within 20 seconds.
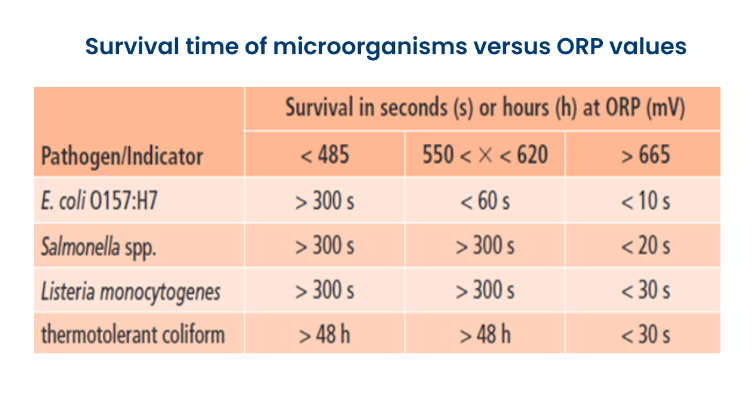
*650-700 mV = 3-5 ppm free chlorine
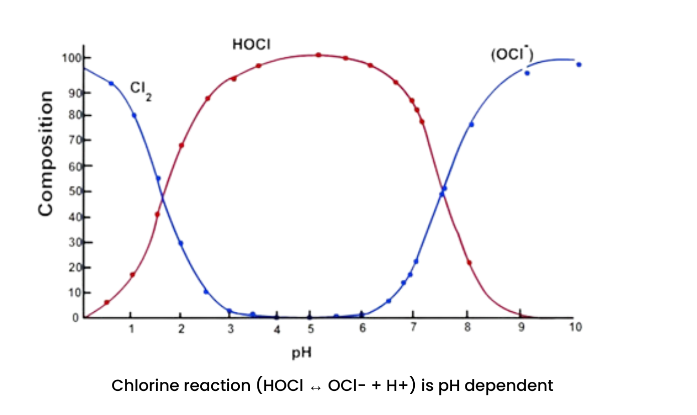
Chlorine is pH dependent. At a pH 5-6, the free chlorine is nearly 100% hypochlorous acid (HOCl) - very effective at killing bacteria. As the pH drops below 4, it starts to convert to Cl2 (chlorine gas). Above pH 6, it starts to convert to the hypochlorite ion (OCl-), which acts predominantly as an oxidizer. Water that is at a pH ≥ 7 may need to be lowered for the chlorine to be at the optimum efficiency as biocidal.

What organic acids do to control bacteria?
We need to know the mode of action of organic acids:
- bacteriostatic effect (ability to reduce the pH of the water by dissociation of its molecules – each molecule donates an H+)
- bactericidal effect (intracellular effect of the undissociated molecules of organic acids – pH reduction, depletion of bacteria energy, interference of DNA replication).
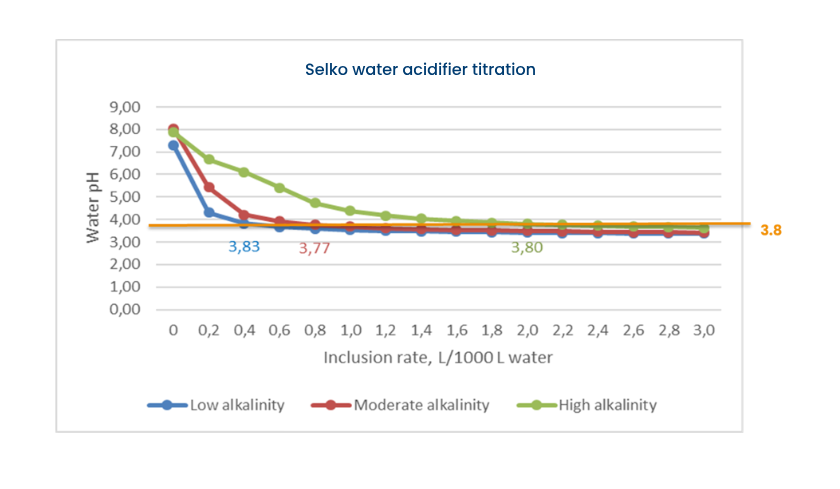
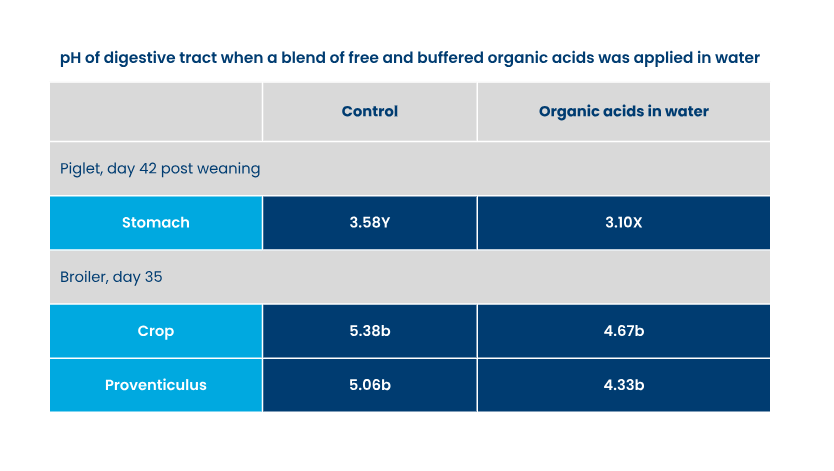
Free acids cause a sharp decrease of the water pH, limiting their inclusion level. As higher doses could impact water intake. Water pH should stay above 3.5-3.7 (depending on animal species) to guarantee a proper water intake. By buffering an acid blend, the pH decrease is less sharp and remains at a higher level. The latter means that products containing buffered acids may be dosed at a higher level, resulting in more undissociated molecules reaching the digestive system of the animals.
Water acidification is not only important for the water itself, but also interesting for young animals, especially in the first-second week of the broiler’s cycle nursery phase. This is because the stomach/proventriculus in this period lacks sufficient capacity to acidify its own content by hydrochloric acid.
Organic acids added via water increase the speed at which the stomach contents reach the optimal pH value, where the proteolytic enzyme pepsin has its optimum efficacy (pH 3-4).
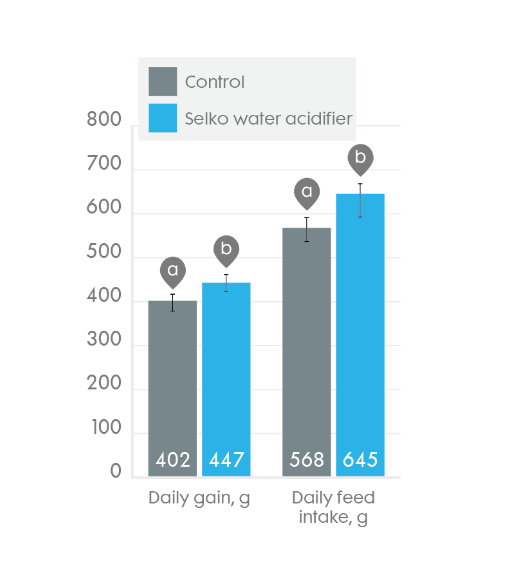
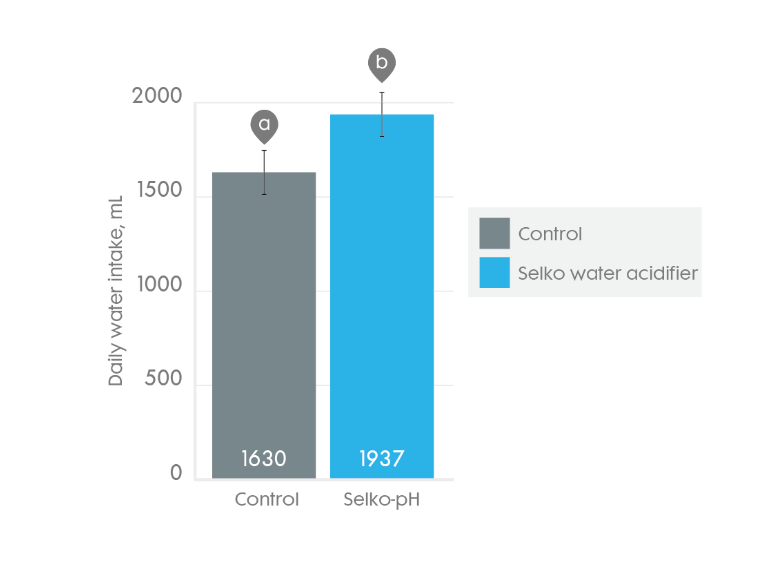
Trouw's water acidifer which consists of blend of free and organic acids improve piglet water intake.
Average daily water intake (with 83% confidence interval as error bars) of weaned broilers with or without Trouw's water acidifier for 42 days. Bars within measurements with different superscripts differ (p < 0.05).
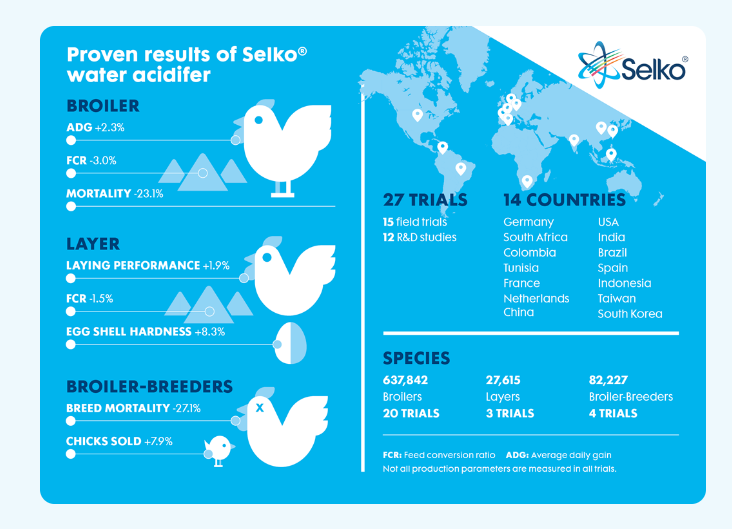
A compilation of 36 R&D/commercial studies (16 with piglets and 20 with broilers; conducted in collaboration with research facilities, universities and farmers in different countries, thereby covering different production systems, climate, and housing conditions) show that buffered organic acids improve the zootechnical performance broilers.
The use of organic acid blends designed to support animal health and efficient production can help support producers’ businesses during all times.
Trouw's water solution
Selko pH
Selko-pH is part of the health programme that focuses on keeping a microbial balance, reducing antibiotic use and enhancing animal performance.